Hemodynamics in large blood vessels
Effects of mechanical stimuli on the growth of Abdominal Aortic Aneurysms
An abdominal aortic aneurysms is a local abnormal dilatation that develops in the aorta, the largest artery that carries blood from the heart to the rest of the body (Figure 1). This vascular disease has become a major health issue that predominantly affects men over 60 years of age. In most cases, the growth of the aneurysm remains unnoticed, until rupture occurs, rupture being a life-threatening event with mortality rates as high as 80%.
Figure 1: Sketch of an abdominal aortic aneurysm. It develops along the abdominal aorta, below the arterial bifurcation to the kidneys.
Over the last decades, studies have shown that the mechanical stresses
exerted by the blood flow have an important effect on the cells
comprised in the arterial walls and on the circulating cells. Mechanical forces
have the potential to stimulate changes in the cell shape, orientation,
secretion, rate of apoptosis and even gene expression. The
goal is therefore to better understand the mechanisms responsible for
the development of vascular diseases by studying the interaction between
mechanical forces and biochemical processes that occur in the cells.
In order to estimate the role that mechanical
stimuli play in the growth mechanisms of the aneurismal dilatations, we aim
at determining the spatial and temporal distributions of the forces induced by
the blood flow and characterising their evolution as the aneurysm grows.
A parametric study has been conducted in axisymmetric and non-axisymmetric models of aneurysms, whose
dimensions have been systematically increased. The flow field inside rigid
models of aneurysms has been measured using Particle Image Velocimetry (PIV)
under the same pulsatile flow conditions as the ones measured in the abdominal
aorta in a healthy patient at rest (collaboration with the Department of
Mechanical and Aerospace Engineering at University of California, San Diego –
USA and with the LadHyX at the Ecole Polytechnique – France). The interactions
between the pulsatile flow and the compliant walls have been simulated
numerically using a finite-element code, LifeV (collaboration with INRIA at
Rocquencourt – France).
Figure 2: (a) Spatial distribution of velocities measured by Particle Image Velocimetry (PIV) in a medium size aneurysm at 3 consecutive instants of time. The flow detaches from the wall around the peak systole (time when the heart expulses the peak flow rate). A vortex ring forms and impinges on the downstream wall, leading to low wall stresses upstream and high wall stresses downstream. (b) Effects of the vortex structures on the trajectories of cells, calculated by Lagrangian particle tracking.
The major event is the flow separation that occurs even at early stages (dilatation ≤ 50%) as the
flow decelerates. In axisymmetric aneurysms, a large vortex ring forms and
impacts the downstream aneurysm wall (Figure 2a). Two regions with distinct
patterns of wall shear stresses (WSS) have been identified: an upstream region
of flow detachment, with low oscillatory WSS, and a downstream region of flow
reattachment, where large negative WSS are produced as a result of the impact
of the vortex ring. For non-axisymmetric aneurysms, a hairpin vortex is shed,
which stays attached to the wall with the smallest curvature. As the vortex
detaches, it rotates and impinges on the latter wall. The wall with the largest
curvature is, however, subjected to quasi-steady reversed WSS of very low
magnitude.
Lagrangian tracking of blood cells inside the different
models of aneurysms shows a dramatic increase in the cell residence time as the
aneurysm grows. While recirculating, cells experience high shear stresses close
to the walls and inside the shear layers, which may lead to cell activation
(Figure 2b). The vortical structure of the flow also convects the cells towards
the wall, increasing the probability for cell deposition and ipso facto for the
formation of an intraluminal thrombus.
Hemodynamic measurements
An essential stage in head and neck microsurgical reconstruction is the choice of
recipient vessels. To make relevant choices, surgeons must rely on accurate
imaging techniques. The objective of the study is to examine the feasibility of
Phase-Contrast sequences to conduct the pre-operative tests without injection
and provide precise radio-anatomical data over the entire vessel region
(Bettoni et al. 2017). The challenges were the large velocity range, the lack
of contrast, and the large spatial resolution needed to image vessels below 5
mm in diameter.
Thirty-one healthy volunteers were included in an MRI prospective study (Figure 3). The
anatomical and morphometric characteristics of the collaterals of the external
carotid artery were determined associating 3D PCA and 2D Cine MRI-PC sequences
(average protocol duration time of 49 min±4 min). The average diameter was
measured to be 2.1±1.4 mm for the superior thyroid artery, 2.2±1.1 mm for the
lingual artery, 2.7±1.6 mm for the facial artery, 2.6±1.4 mm for the internal
maxillary artery, and 2±1.4 mm for the superficial temporal artery. With a
vessel identification success rate of 98%, the study showed that Phase Contrast
MRI allowed non-invasive and non-operator dependent anatomical analyses of
small calibre vessels without the use of agent contrast. It also proved that
the designed sequences could be used on patients and provided valuable
pre-operative information for head and neck surgery.
 b
b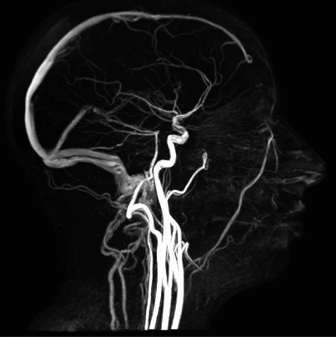
Figure 3: In vivo radio-anatomical pilot study of the feasibility analysis of non-injected phase-contrast MRI sequences for the mapping of the external carotid branches: (a) 32-element head coil and (b) head and neck blood vessels (Bettoni et al. 2017)
Collaborators
Prof. Juan Lasheras, University of California San Diego, La Jolla
Dr. Jean-Marc Chomaz, Ecole Polytechnique, Palaiseau
Dr. Steven Sparks.
Dr Jérémie Bettoni, Maxillofacial surgery, University Hospital of Amiens, CHU Amiens-Picardie. PhD Thesis.
Dr Gwénaël Pagé, BioflowImage laboratory, Université Picardie Jules Verne. PhD Thesis.
Dr Stéphanie Dapké, Maxillofacial surgery, University Hospital of Amiens, CHU Amiens-Picardie.
Dr Olivier Balédent, BioflowImage laboratory, Université Picardie Jules Verne.
Prof. Bernard Devauchelle, Maxillofacial surgery, University Hospital of Amiens, CHU Amiens-Picardie.
Prof. Sylvie Testelin, Maxillofacial surgery, University Hospital of Amiens, CHU Amiens-Picardie.
Prof. Jean-Marc Constans, Radiology Department, University Hospital of Amiens .
Faire Faces Institute, Amiens