Minimally invasive therapeutic techniques
Vascular embolization by glue injection
Vascular embolization is a minimally invasive treatment used to selectively eliminate or stop the vascular supply to specific body areas. One technique consists of navigating a microcatheter into the targeted blood vessel and injecting an embolic agent which reacts in contact with blood (Figure 1). Cyanoacrylate-based embolic glues are the main liquid adhesives used for vascular embolization owing to their low viscosity, good penetration ability and low tissue toxicity. To enable its detection once injected, the glue is mixed with a radio-opaque contrast agent such as the Lipiodol® iodized oil. Although the technique is commonly used to treat arteriovenous malformations, vessel haemorrhage, tumors, etc, there is very little information on the dynamics of the injection process in complex blood flows or on the polymerization kinetics of the glue-Lipiodol mixture. Consequently, safe occlusion is difficult to achieve, even in the hands of experienced radiologists.
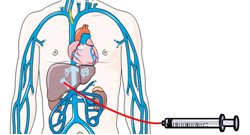
Figure 1: Glue is injected percutaneously to embolizethe part of the liver to be resected.
The objective of the study is to investigate the coupling between the various phenomena governing the process. When vessels are embolized by glue injection, two primary physical phenomena predominate: one is the fluid dynamics of the injection of a fluid into another non-miscible co-flowing fluid, the other is the polymerization kinetics of the embolic glue. Both phenomena, which are both strongly coupled, have characteristic time constants that strongly depend on the properties of the glue mixture and of the conditions of injection.
1. Study of glue injection in a confined external flow
 b
b
Figure 2: In vitro experiment simulating the injection of a non-miscible fluid in a co-flow. (a) Dripping regime. (b) Jetting regime.
From a fluid mechanics point of view, embolization is governed by the dynamics of two co-flowing
immiscible fluids, which is coupled with the kinetics of glue polymerization.
Experimental investigations on drop formation in liquid-liquid systems have
shown the existence of two regimes, dripping and jetting. Most studies have
focused on dripping in unconfined environment. We aim at characterizing the
flow topology during portal vein embolizationby
studying experimentally the confined liquid-in-liquid injection, neglecting
glue polymerization as a first approach.
An experimental setup has been designed to create and visualize the co-flow of
two immiscible fluids (Sandulache et al. 2012). The dispersed phase is
injected through a capillary tube into the continuous phase, which flows
steadily in a straight cylindrical tube. The experiment is conducted in
similarity with the physiological case.
We find that both regimes may occur during hepatic embolization
(figure 2). Large drops form periodically during the dripping regime. During
jetting, a jet forms instead at the tip of the catheter and destabilizes after
a short distance, leading to the formation of small drops. The dripping-to-jetting
transition is governed by the ratio of the inertial forces of the injected
phase to the interfacial forces with the external phase (Weber number).
During dripping, the drop size is only dependent upon the ratio of the viscous
forces of the external face to the interfacial forces (capillary number). An
analytical model has been developed to predict the drop size evolution; good
agreement is found with experimental data. During jetting, the drop size tends
towards a constant value, which is a function of the jet diameter.
2. Study of the polymerization kinetics of cyanoacrylate glues
The objective was then to quantitatively investigate the physical properties of two
n-butyl cyanoacrylate (nBCA) glues (Glubran 2 and Histoacryl) mixed with an iodized oil (Lipiodol)
at various concentrations (Li et al. 2017a).
We have shown that a homogeneous solution results from the mixing of the glue and Lipiodol (Figure 3), and that the viscosity, density and
interfacial tension of the mixture increase with the proportion in Lipiodol.
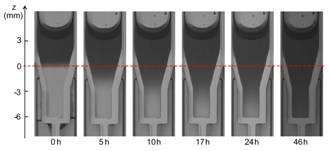
Figure 3: Time evolution of nBCA glue put in contact with Lipiodol: the darkening of the system over time shows that the two liquids are miscible. The dashed line shows the initial position of the interface.
We have designed a new experimental setup to systemically characterize the polymerization kinetics of a glue mixture upon contact with an ionic solution. We observe that the whole polymerization process includes two phases (Figure 4): an interfacial polymerization that takes place at the interface as soon as the two liquids are in contact and that has a characteristic time scale of the order of the minute; a volumetric polymerization during which a reaction front propagates within the mixture bulk with a characteristic time scale of the order of tens of minutes. The polymerization rate, front propagation speed and volume reduction increase with the glue concentrations. It is the first time that such comprehensive results are obtained on liquid embolic agents.
 b
b c
c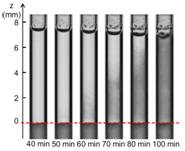
Figure 4: (a) Change in opacity observed in the ionic solution in the case of a Glubran2-Lipiodol (G-L) mixture (glue concentration of 50%): the darkening in the ionic solution reveals the polymerization of a G-L film. (b) Scanning electron micrograph of a G-L film removed from the tube. (c) Time evolution of G-L mixture (glue concentration of 50%) in contact with the ionic solution: the darkening in the glue mixture over time shows the propagation of a polymerization front upwards from the interface (Li et al. 2017a).
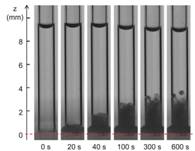 b
b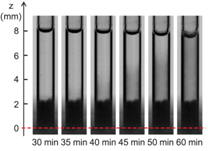
Figure 5: Time evolution of nBCA glue put in contact with Lipiodol: the darkening of the system over time shows that the two liquids are miscible. The dashed line shows the initial position of the interface.
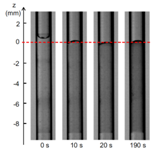
Our next objective of the study has been to determine the influence of plasma proteins in the polymerization reaction (Li et al.2017b). A triggering solution containing bovine serum albumin (BSA) and the main blood ions is used as a model of plasma. The polymerization kinetics of Glubran 2-Lipiodol mixtures is measured upon aspiration in a capillary tube and contact with the proteinaceous solution. Having varied the glue and protein concentrations, we show that glue-Lipiodol mixtures with concentrations larger or equal to 25% polymerize when put in contact with an ionic solution containing at least 4% of BSA. The reaction is decomposed into two phases: a fast zwitterionic polymerization induced by the BSA molecules followed by a slower polymerization phase (Figure 5). The reaction speed and extend of the solidification region mostly depend on the glue concentration. The time for the glue solution to polymerize over a 1 mm thickness varies from 5 s for pure glue to about 1 min for a 50% glue concentration, and 10 min for a 25% glue mixture. It is the first time that the kinetics of the two polymerization reactions is quantified for Glubran 2, which will provide the information needed by interventional radiologists to optimize the planning of endovascular glue injection.
Collaborators
Dr. Olivier Balédent, Service de Biophysique et Traitement de l’Image, CHU Amiens.
Dr. Roger Bouzerar, Service de Biophysique et Traitement de l’Image, CHU Amiens.
Dr. Thierry Yzet, Département de Gastroentérologie, CHU Amiens.
Dr. Audrey Fohlen, Unité de Radiologie Interventionnelle, CHU Caen.
Prof. Romaric Loffroy, Département de Radiologie Diagnostique et Thérapeutique, CHU Dijon.
Dominique Haye, Plate Forme Mécatronique, Saint Quentin.
Guerbet,Aulnay-sous-Bois.
GEM srl.
Mihai-Cristinel Sandulache, In vitro characterization of the endovascular technique of embolization by injection of surgical glue, PhD Thesis, UTC, October 2011.
Bilal Merei, Simulation simulation of the injection of a non-polymerizing fluid in a hepatic vein, Master Thesis, UTC, September 2010.
Océane Lançon, In vitro study of the injection of a surgical glue for hepatic embolization, Master Thesis, UTC, September 2012.
Giorgio Davico, Numerical study of the embolization of arteriovenous malformations by glue injection, Master Project, Politecnico Di Turino (Italy).
Mathieu Delpech,Study of the volumetric polymerization of surgical glues, Master Project, UTC, September 2015.
Yongjiang Li, Embolization by glue injection, PhD Thesis, UTC, April 2017.
Audrey Kalic, Engineer, UTC.
Hemodynamics in arterio-venous fistula (AVF)
An arteriovenous fistula (AVF) is a surgical vessel connection between an artery and a vein. It is created in end-stage renal disease to provide adequate blood access for hemodialysis (Figure 6).
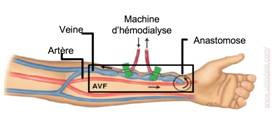 b
b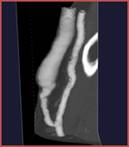
Figure 6: (a) Forearm anatomy in a patient with an arterio-venous fistula. (b) Patient-specific geometry used in the fluid-structure interaction numerical simulation (Decorato et al. IJNMBE 2014)
1. Numerical modelling of the fluid-structure interactions in an AVF
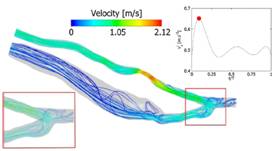
Figure 7: Velocity streamlines at peak systole. The time of measurement is indicated by the red dot on the inlet velocity profile (Decorato et al. IJNMBE 2014).
The objective of the study is to investigate numerically the fluid-structure interactions (FSI) in a patient-specific AVF and analyze the degree of complexity that such a numerical simulation requires to provide clinically relevant information (Decorato et al. IJNMBE 2014). The reference FSI simulation takes into account the non-Newtonian behaviour of blood, as well as the variation in mechanical properties of the vascular walls along the AVF. We have explored whether less comprehensive versions of the simulation could still provide relevant results. The non-Newtonian blood model is necessary to predict the hemodynamics in the AVF because of the predominance of low shear rates in the vein. An uncoupled fluid simulation provides informative qualitative maps of the hemodynamic conditions in the AVF; quantitatively, the hemodynamic parameters are accurate within 20% maximum. Conversely, an uncoupled structural simulation with non-uniform wall properties along the vasculature provides the accurate distribution of internal wall stresses, but only at one instant of time within the cardiac cycle. The FSI simulation advantageously provides the time-evolution of both the hemodynamic and structural stresses. However, the higher computational cost renders a clinical use still difficult in routine.
2. Influence of an arterial stenosis on the hemodynamics within an arteriovenous fistula
We have then focused on the occurrence of arterial stenoses in arteriovenous fistulae (AVF), which was long underestimated. The objective has been to investigate their influence on the hemodynamic conditions within the AVF (Decorato et al. CVET 2014, IJAO 2014). A numerical simulation of the blood flow has been conducted within a patient-specific arteriovenous fistula that presents an 60% stenosis on the inflow artery. In order to find the vessel shape without stenosis and compare the flow conditions with and without stenosis, the endovascular treatment of balloon-angioplasty is simulated by modeling the vessel deformation during balloon inflation implicitly. Clinically, balloon-angioplasty is considered successful if the post-treatment residual degree of stenosis is below 30%. Different balloon inflation pressures have been imposed numerically to obtain residual degrees of stenosis between 30 and 0%. The comparison of the computational fluid dynamic simulations carried out in the patient-specific native geometry and in the treated ones shows that the arterial stenosis has little impact on the blood flow distribution. The venous flow rate remains unchanged as long as thrombosis does not occur: the nominal flow rate needed for hemodialysis is maintained, which is not the case for a venous stenosis. An arterial stenosis, however, causes an increase in the pressure difference across the stenosed region. A residual degree of stenosis below 20% is needed to guarantee a pressure difference lower than 5 mmHg, which is considered to be the threshold stenosis pressure difference.
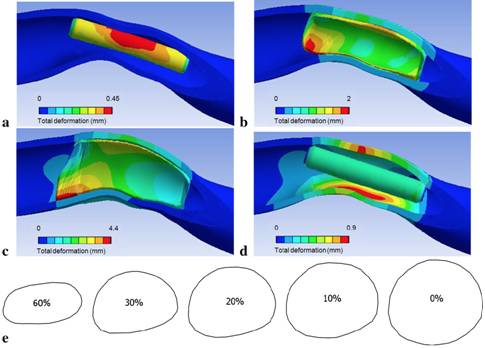
Figure 8: Snapshots of the evolution of the artery shape during the numerical simulation of balloon-angioplasty. (a) Initial configuration. (b) Configuration when the balloon comes into contact with the artery. (c) Configuration at maximum balloon internal pressure. (d Vessel final shape when the balloon is completely deflated. (e) Vessel cross-sections at the throat of the stenosis for the patient-specific (60%-stenosis) and treated geometries (30, 20, 10, 0% residual stenosis).(Decorato et al. CVET 2014).
Collaborators
Dr. Zaher Kharboutl, BMBI,UTC
Dr. Cécile Legallais, BMBI, UTC
Dr. Vanessa Diaz, University College London (UK)
Prof. Gabriele Dubini, Politecnico di Milano (Italy)
Dr. Justin Penrose, ANSYS UK (UK)
Iolanda Decorato, Numerical simulation of the fluid-structure interactions in an arteriovenous fistula: effect of stenting hemodynamics, PhD Thesis, UTC, February 2013.
Tommaso Vassallo, In vitro study of hemodynamics in an arteriovenous fistula, Master Thesis, Politecnico Di Turino (Italy), September 2012.